A landmark study published in the May 31st, 2002 issue of "Science", corroborated observations made by Ayurvedic physicians in India, 2500 years ago. Medical researchers led by Dr. David D. Moore of Baylor College of Medicine, Texas, USA, demonstrated that the gum resin extract of Guggul (Commiphora mukul from the Burseraceae family to which the myrrh of Biblical renown also belongs), a botanical long known for its health benefits, has beneficial effects on cholesterol metabolism at the molecular level.
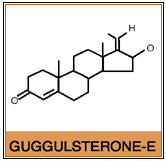
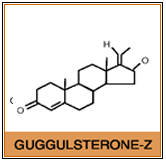
These US researchers unraveled the potential mechanism of action of guggulsterones, the biologically active components of the resin. Guggulsterones were shown to be antagonist ligands for the bile acid receptor FXR, which is an important regulator of cholesterol homeostasis in the body.
Scientific studies on guggul began almost 40 years ago when an Indian researcher, G.V. Satyavati was intrigued by the strong parallels between modern concepts on the etiology of atherosclerosis and obesity, and descriptions in the Sushruta Samhita written in the 5th to 4th century B.C. Traditionally, guggul is used in formulations for the treatment of inflammation, arthritis, cardiovascular conditions and obesity. Supported by scientific research, it has now been rediscovered as a hypolipidemic agent.
Gugulipid®, a registered trademark of Sabinsa Corporation, is the standardized extract of the oleogum resin of Commiphora mukul. This resin is a mixture of diterpenes, sterols, steroids, esters and higher alcohols. The active ingredients responsible for the hypolipidemic health benefits, are the guggulsterones, specifically guggulsterone E and guggulsterone Z.. It is important that guggul extracts be properly standardized and assayed using HPLC methods to ensure that side effects such as rashes and gastrointestinal disturbances associated with crude guggul extracts, do not occur.
In addition to two standardized powder extract products - Gugulipid® granules and Gugulipid® 40 Mesh, containing a minimum of 2.5% guggulsterones Z and E (by HPLC), Sabinsa also offers Gugulipid® Soft Extract, which is standardized for a minimum of 7.5% guggulsterones Z and E (by HPLC). This extract is manufactured using state-of-the-art supercritical carbon dioxide extraction, which eliminates the likelihood of residual solvents in the product.
An IND (Investigational New Drug) study approved by the USFDA seeks to further validate the hypolipidemic health benefits of Gugulipid®, Sabinsa’s trademarked extract manufactured by WHO/GMP certified Sami Labs Ltd.
US Patent 6,436,991 dated August 20, 2002, granted to Sabinsa Corporation, covers the antioxidant and cancer chemopreventive roles of constituents of Gugulipid®, This invention, titled "Compositions for prevention and treatment of abnormal cell growth and proliferation in inflammation, neoplasia and cardiovascular disease", further unravels the multifaceted healthful benefits of this increasingly popular phytonutrient.








