Acne vulgaris is a chronic inflammatory disorder of the pilosebaceous unit, the hair follicle and its associated sebaceous glands, located on the face, chest, and back. Several factors contribute to the pathogenesis of acne. They include sebum, inflammation, abnormal follicular differentiation, and Propionibacterium acnes. Acne has been reported to cause psychological and social disability amongst its sufferers, especially young adults.
Conventional drugs, systemic and topical antibiotics, have been used to treat acne. However, they have side effects that may cause problems with long-term use. New, effective treatments suitable for long-term therapy have been sought for Acne vulgaris.
Ayurveda is an ancient branch of medicine that has been used for a long time in the Indian system of medicine. It has been called the "science of life (‘Ayur’ means life and ‘Veda’ means knowledge). Several Ayurvedic formulations have been used to treat acne; however, their effectiveness has not been tested in controlled clinical trials. Paranjpe et al. evaluated 4 Ayurvedic treatments for acne in a randomized, double-blind, placebo-controlled, clinical trial.
The four Ayurvedic formulations are known in vernacular as 1) Sookshma Triphala, 2) Thiostanin, 3) Shankhabhasma Vati, and 4) Sunder Vati. Sookshma Triphala is comprised of minerals and the fruits of three botanicals combined to prepare triphala, Terminalia chebula ("Harad"), Terminalia belerica ("Bahera"), and Emblica officinalis ("Amla"). Thiostanin is comprised of minerals, the fruits of T. belerica, T. chebula, and Emblica officinalis, Commiphora mukul ("Guggul"), nd the roots of Plumbago zeylanica, and Picrorrhiza kurroa a ("Kutaki"). Shankhabhasma Vati consists of conch shell, and Sunder Vati consists of Holarrhena antidysenterica ("Kuda"), Emblica officinalis, Embelia ribes ("Baberang"- Hindi, "Vidanga"- Sanskrit), and Zingiber officinale ("Adrak"- Hindi, Ginger- common name). (The names given in the parentheses are in Hindi unless otherwise noted.) Each formulation was prepared into 250 mg tablets for administration.
Participants of the study included eighty-two patients (40 males and 42 females) with moderate Acne vulgaris of the face manifested by at least 10 papules or pustules and at least 5 open or closed comedones. (papule- small, raised abnormality or spot on the skin.; pustule- a small blister on the skin containing pus.; comedone- a plug of sebum and keratin in the outlet of the skin’s sebaceous gland that is also known as a blackhead.) Of the 82 patients, 66 completed the study. All subjects discontinued the use of topical and systemic acne treatments and medications at least 2 weeks before entry into the study. They were randomly divided into 5 groups- Sookshma Triphala, Thiostanin, Placebo, Shankhabhasma Vati, and Sunder Vati. None of the groups significantly differed concerning sex, age, diet, smoking status, complexion, use of makeup, allergies, or status of acne. Depending on which group they belonged to, patients took two tablets of their specified treatment three times a day with water and after meals for a period of 6 weeks.
Every 2 weeks the subjects’ skin was evaluated by one of the study’s investigators. The investigator evaluated the subjects by 2 parameters: (1) the exact number of lesion types (papular, pustular, nodulystic lesions, and open or closed comedones) on the face from ear to ear, (2) comparison of patient’s facial acne after treatment with his/her initial appearance before treatment rated on a scale from excellent to worse.
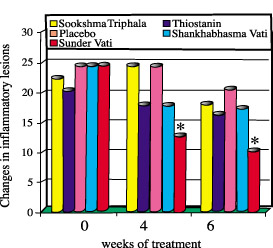
Figure 1. Changes in inflammatory lesions during treatment. (*p<0.01) Of the Ayurvedic formulations, Sunder Vati was the only formulation found to be orally effective in the treatment of Acne vulgaris. The lesions of the Sunder Vati group were significantly reduced. This group also showed an excellent response to an overall change in facial acne.
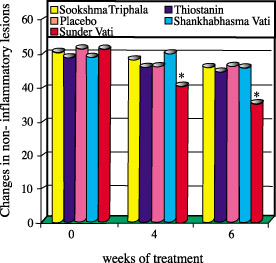
Figure 2. Changes in non-inflammatory lesions during treatment. (*p<0.01) Source: Paranjpe, P. and Kulkurni, P.H. (1995) J. Ethnopharmacol. 49, 127-132. Sabinsa Corporation supplies standardized extracts of Terminalia belerica (min. 40% tannins), Terminalia chebula (min. 40% tannins), Emblica officinalis (min. 40% tannins), Triphala (combination of the first 3 standardized extracts), Embelia ribes (min. 5% embelin), Gugulipid® (Commiphora mukul- dry extract: min. 2.5% guggulsterones Z & E and soft extract: min. 7.5% guggulsterones Z & E), Picroliv® (Picrorhiza kurroa- min. 4% kutkin and 8-10% bitter principles), and Zingiber officinale (dry extract: min. 5% gingerols and soft ectract: min. 20% gingerols. Powders are also available of T. belerica, T. chebula, Triphala, E. officinalis, and P. kurroa. Please contact Sabinsa for information about these products and the other products of our nutritional, cosmeceutical, and functional food lines.
Sabinsa Corporation will participate in the following tradeshow events in January 2001.
Sabinsa Corporation will participate in the following trade show events in March 2001.
January 29-31, 2001:
Nutritional 2001(Anaheim, CA)
Booth # 309








